Abstract
One of the major diseases of Solanaceae family is early blight caused by Alternaria solani which lowers the yield with reduction of plant health and seed quality. The current study assessed the presence of secondary metabolites in the extracts of selected weeds such as Cannabis sativa, Parthenium hysterophorus, and Lantana camara using phytochemical screening and their antifungal interactions with the target pathogen Alternaria solani. The results of phytochemical screening showed that P. hysterophorus was particularly rich in amino acids, which were not found in the aqueous and ethanol extracts of C. sativa and L. camara. Proteins were detected in all extracts except those from P. hysterophorus. Tannins were absent only from L. camara, but present in the other extracts. P. hysterophorus also had a substantial amount of flavonoids, which were absent from the ethanol extract of L. camara but present in other extracts. Phytosterols were found in every extract of C. sativa, L. camara, and P. hysterophorus. Food poison method and Agar disc diffusion method were used to investigate the antifungal behavior of weeds extracts against Alternaria solani. The food poison method produced significantly better results than the agar-disc diffusion method. However, the order of effectiveness of weeds using these two methods were: C. sativa > L. camara > P. hysterophorus. Moreover, maximum inhibition was observed in the aqueous extract of C. sativa after seven days of incubation. The outcomes of this study could lessen the need for artificial fungicides and aid in controlling the emergence of disease resistance and hold substantial significance in the realm of sustainable agriculture and disease management.
Keywords: Agar disc diffusion method, Disease management, Food poison method, Secondary metabolites
INTRODUCTION
The Solanaceae family includes several economically important crops, making it one of the most valuable plant families for vegetable and fruit crops (Muriel and Moreels, 2022). Solanaceae family is approximately affected by 60 phytopathogens, including bacteria, fungi, viruses, and oomycetes, which can infect plants and cause severe diseases and produce major losses (Jones et al., 2017). One of the most important diseases of Solanaceae family is early blight caused by A. solani i.e., an asexual, soil inhabiting necrotrophic fungus, thrives in warm, humid environments and disseminates through airborne spores causing devastating foliar diseases. This fungus is significantly important because it cause disease in many economically cash crops such as in tomatoes, potatoes, pepper, cauliflower, broccoli, cereals, apple and many other ornamentals plants (Thomma, 2003). Currently the yield losses of Solanaceae family due to Alternaria are estimated from 50% to 86% losses in fruit yield and 20% to 40% losses in seedling establishment.
The prevailing methods to manage early blight predominantly revolve around chemical fungicides. However, these conventional approaches pose multifaceted challenges. Failure to control this disease can cause reduction in yield (Malik et al., 2014). Despite their initial efficacy, their long-term use raises environmental concerns due to their nonspecific action, health hazards, escalating production costs, and the development of resistance among strains of A. solani (Mandeel, 2020). As a consequence, there is an urgent need for sustainable and eco-friendly alternatives to mitigate this agricultural menace.
The most effective way to control this disease is to use resistant cultivars. However, farmers who aim for a large yield are more likely to grow some types that might not be as disease-resistant. In addition to harming user and consumer health, the widespread and unintended use of fungicides frequently causes major environmental issues. Therefore, reducing the amount of chemicals used to treat disease is essential. To manage the early blight of tomatoes in an environmentally friendly manner, an evaluation of several new agrochemicals, plant extracts, and bio-agents against A. solani has been undertaken (Bais et al., 2019).
The presence of phytochemicals in weed plants makes them useful for treating human diseases and curing numerous disorders. Phytochemicals are found in all sections of a plant, including the stem, leaves, root, and fruit, and they serve as defensive mechanisms, protecting the plant against a variety of diseases. Secondary metabolites are natural plant compounds that are byproducts of main metabolites such as chlorophyll, lipids, amino acids, and carbohydrates. Plants have active secondary metabolites such as saponins, tannins, glycosides, terpenoids, flavonoids, steroids, and alkaloids (Pandey and Tripathi, 2019). The primary function of these phytochemicals is to offer long-term immunity in the form of resistance against various disease-causing microorganisms. Phytochemical screening is essential for identifying and isolating chemical substances in plants to enhance their medicinal potential (Pandey and Tripathi, 2019).
To prepare plant extracts for treating infections, it is necessary to identify the existence of active phytochemical components in the plants. Thus this study deals with the qualitative phytochemical screening of three weeds plants which are, P. hysterophorus, referred to as parthenium weed locally known as Chatak Chandni, C. sativa commonly known as Bhaang, L. camara also known as Panj Phulli.These are herbaceous plants that grow rapidly. P. hysterophorus is a member of the Asteraceae family. It is an invasive weed originally native to northeastern Mexico, partheniums was once confined to America but now has spread to more than 50 nations worldwide (Adkins et al., 2019). Some of the key traits influencing invasiveness and competitiveness include a fast growth pattern, the capacity to acquire resources, resistance to abiotic stressors, allelopathic potential, and phenotypic flexibility (Nguyen et al., 2017). This weed is suspected of causing substantial yield losses, mutations in livestock and humans, and allergic respiratory issues caused by its allelopathic influence. Allelopathic chemicals released by exotic weed species can inhibit nearby competing plants (Motmainna et al., 2021; Qu et al., 2021). The seeds can easily dispersed by water (Mao et al., 2019) or by strong wind (Mao 2020). Methanolic extract of P. hysterophorus revealed effective antifungal potential against Candida kefyr, Candida albicans and Aspergillus niger (Malarkodi et al., 2013).
C. sativa is a member of Cannabaceae family and is native to Eastern Asia, has spread worldwide because of its therapeutic properties (Berardo et al., 2024). Cannabis originated from the alpine foothills of the Himalayas and is found in different altitudes and habitats. Reports indicate that it is widely used for numerous reasons as a drug to cure illness. Antibacterial cannabinoids such as cannabigerol, cannabichromene, cannabinol, Δ9-tetrahydocannabionol, Cannabidiol and other chemical compounds alkaloids, flavonoids, phenolics and terpenoids are present in it (Khan and javaid, 2020; Lone and Lone, 2012). Different concentrations of methanolic leaf extracts of C. sativa show antifungal potential against Aspergillus flavipes (Khan and javaid, 2020). Antimicrobial activity of this weed is reported against Staphylococcus, Bacillus subtilis, Pseudomonas aeruginosa and Escherichia coli (Ali et al., 2012).
L. camara is a member of Verbenaceae family. This weed is native to tropical America, but now has spread to more than 60 countries worldwide (Kato and Kurniadie, 2021). L. camara also possess allelopathic compounds which can suppress the growth of nearby plants. Extracts of lantana has provided promising results as alternative to conventional fungicides. The presence of several bioactive phytocompounds in the plant contributes to its medicinal potential (Girish, 2017).
Antifungal activity of C. album was observed against fungus Fusarium oxysporum causing basal rot disease in onion. Aqueous extract of L. camara can suppress or inhibit the growth and germination of various plants such as Brassica juncea, Cucumis sativus, Phaseolus mungo, Raphanus sativus, Vigna unguiculata, Cicer arietinum (Ahmed et al., 2007). Although many research works have so far been done on the inhibitory effects of many chemical fungicides on agricultural crops throughout the world (Mandeel, 2020), our present work was an ecofriendly attempt to explore the inhibitory effects of different extracts (derived from different weeds) for the management of early blight caused by Alternaria solani in tomato plants (which belongs to Solanaceae family).
MATERIALS AND METHODS
Collection and preparation of plant material
Fresh, leaves of P. hysterophorus, C. sativa and L. camara were collected from different areas of Zafarwal, Kingara and Shakargar. Leaves were thoroughly washed with tap water, and shade dried for 15 to 20 days. The dried leaves were grounded into fine powder and stored at 4°C. Following the maceration technique, 10 g of powdered leaves were soaked in 100 ml of methanol, ethanol, or aqueous solvent in 250 ml conical flask separately. These flasks were covered with cotton plug, then sealed properly with paraffin and left at room temperature for three days and the extracts were filtered with Whatman No. 42 filter paper (Ahmed et al., 2007).
Qualitative phytochemical screening of selected weeds
In order to assess the antifungal potential of the plants, the resulted weed extracts were subjected to various qualitative phytochemical screening tests. This analysis helped in detection of various bioactive metabolites using standard protocols. All experiments were performed in triplicates for each of plant extracts (Olikh et al., 2023).
Detection of amino acid
Ninhydrin Test was used to detect the Amino acid by adding 10 drops of Ninhydrin solution (10 mg Ninhydrin in 200 ml acetone) into 2 ml of each plant extract. Purple color confirmed the presence of amino acid (Olikh et al., 2023).
Detection of flavonoid
Ferric chloride test was used – in a test tube 1 ml of 10% FeCl3 was slightly added into the 1 ml of each plant extract. The Mixture was shaken for 3 minutes by hand. Appearance of dull green/reddish brown color indicated the presence of flavonoid (Shaikh and Patil, 2020).
Detection of phytosterols
Salkowski test was applied – 1 ml of each plant extract was added into 1 ml of conc. H2SO4. Solution was shaken vigorously by hand and cooled at room temperature. Appearance of red color indicated the presence of Phytosterols (Olikh et al., 2023).
Detection of protein
For protein detection, 1 ml of each weed extract was added in 1 ml of conc. HNO3. Afterwards it allowed to cool down at room temperature and 1 ml of 40% NaOH was also added into test tubes. Dark yellow/ orange – red color was observed for the presence of protein in tested samples. (Olikh et al., 2023).
Detection of tannins
For Tannins, 0.5 ml of each plant extract was added in 4 ml of 10% NaOH solution. The mixture was shaken by hand for 5 minutes and left at room temperature for the emulsion formation which indicated the presence of tannins in solutions (Shaikh and Patil, 2020).
Collection of fungus
The identified strain of Alternaria solani (accession No FCBP-PTF-831) was collected from the First Fungal Culture bank of Pakistan (FCBP), Institute of Agricultural Science (IAGS), University of the Punjab, Lahore, Pakistan. This fungus was stored at 4 °C until used.
Antifungal activity of the selected weed extracts
To evaluate the effect of weed extracts against A. solani, Food Poison Method and Agar Well Diffusion Methods were used. For Food poison method, 2 ml of each prepared plant extract was mixed with 10 ml of sterilized PDA in petri dishes and the content was agitated into circular motion to mix the extracts in PDA homogenously. These petri dishes were inoculated with 5 mm diameter agar plugs containing active mycelium (6-7 days old culture) of A. solani in the center of plates and incubated at 28 ± 1ºC for 7 days (Gupta et al.,2011; Pal et al., 2013). Agar disc diffusion method was performed by using the method adopted by Ahmed et al. (2017). For this method, 20 ml of PDA was poured to petri dishes, after solidification 5 mm of fungus culture was added to these petri dishes. Whatman No. 3 filter paper of 6 mm disc was soaked in each weed extract for 10 minutes and carefully placed on the agar surface. Afterwards, these dishes were incubated at 28 ± 1ºC and the diameter of inhibition zone was measured (Ahmad et al., 2017). The experiments were performed in triplicates with suitable control. Plates with PDA without plant extracts served as control. After 3, 5 and 7 days of incubation, diameter of fungal mycelium (mm) was measured and recorded. Antifungal activity of each leaf extract was measured by using a formula:
% inhibition= (dc-dt)/dc × 100
dc= is the average increase in fungal growth in control
dt= is the average increase in fungal growth in treated
Statistical Analysis
All the data obtained from the experiments was analyzed using analysis of variance (ANOVA) for comparison of the means of treatment. Fisher’s least test (LSD) tests was used for comparison and separation of means at 0.05% level of significance.
RESULTS
Phytochemical screening of Cannabis sativa, L. camara and P. hysterophorus extracts
Three extracts of selected weeds i.e. ethanol, methanol and aqueous were subjected to phytochemical screening for the detection of amino acids. Appearance of purple color showed the presence of amino acids in the extracts. Qualitative results showed the presence of amino acids in all the extracts of P. hysterophorus but also showed the absence of amino acids in the aqueous and ethanol extracts of C. sativa and L. camara respectively. Extracts of selected weeds were also tested to indicate the presence of flavonoids. Flavonoids presences was detected by the appearance of green color precipitations. Green color was displayed in all of the extracts of C. sativa and P. hysterophorus but found absent only from the ethanol extract of L. camara. The presence of proteins in any extracts is detected by the display of Dark yellow/ orange-red coloration. Results showed that all extracts of C. sativa and L. camara indicated the presence of proteins as all extracts revealed the required color except the aqueous extracts of P. hysterophorus. Tannins are detected by the formation of emulsion in the solution. Results in Table 1 showed that C. sativa and P. hysterophorus were rich in tannins as all extracts confirm the presence of tannins in them while they were absent from the ethanol extract of L. camara. Presence of red color indicated phytosterols in the solution. Results showed the presence of phytosterols in all the extracts of C. sativa, L. camara, and P. hysterophorus (Table 1).
Antifungal activity of selected weeds
Based on the phytochemical analysis results, three selected weeds were tested for antifungal activity against Early blight causing fungi (A. solani) using the food poison method and the agar disc diffusion method. The In vitro results of all extract of selected weeds showed statistically significant antifungal activity against the A. solani. Notably, the Food Poison Method inhibited A. solani mycelial growth the most effectively than the Agar Disc Diffusion Method. However, the aqueous extracts have the highest antifungal activity compared to the methanol and ethanol extracts in both methods.
The lowest mycelial growth rate due to antifungal activity was as follows: C. sativa (1.69 cm - 4.09 cm) > P. hysterophorus (2 cm - 4.66 cm) > L. camara (1.92 cm - 4.57 cm) in ethanol extracts; C. sativa (1.69 cm - 3.85 cm) > L. camara (1.7 cm - 4 cm) > P. hysterophorus (1.9 cm - 4.57 cm) in methanol extracts and C. sativa (0.55 cm - 2.57 cm) > L. camara (1.5 cm -3.36 cm > P. hysterophorus (1.87 cm - 4.25 cm) in aqueous extracts (Figure 1).
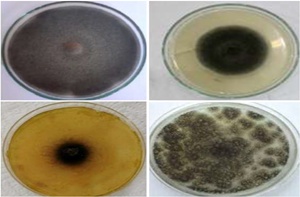
The qualitative results in Figure 2 also showed that disc of extracts inhibited mycelium growth of A. solani by the maximum range inhibition post seven days of inoculation.
In contrast, The Agar Disc Diffusion Method’s effectiveness was ranked as follows: C. sativa > L. camara > P. hysterophorus for ethanol extracts; C. sativa > P. hysterophorus > L. camara for methanol extracts and C. sativa > L. camara > P. hysterophorus for aqueous extracts. These suppression and inhibition in A. solani mycelium’s growth for all weed extracts were clearly visible and consistent in Figure 3. Moreover, maximum inhibition after seven days of inoculation was observed in aqueous, methanol and ethanol extracts of C. sativa 2.05 cm - 3.97 cm, 2.07 cm - 4.1 cm and 2.18 cm - 4.18 cm respectively as compared to other weeds (Figure 1).
DISCUSSION
Solanaceous crops such as Tomatoes, potatoes and peppers are essential in global agriculture, but their susceptibility to early blight, primarily caused by the necrotrophic fungus A. solani, remains a significant concern. This disease poses considerable economic threats, leading to defoliation, decreased photosynthesis, and substantial yield losses in tomato crops (Mandeel, 2020).
This study primarily looked at the antifungal activity of selected weeds extracts against A. solani utilizing two In vitro methodologies. Prior to testing the antifungal properties, a qualitative phytochemical investigation was performed to determine the existence of secondary metabolites in the plants. This research identified five significant compounds: amino acids, proteins, tannins, flavonoids, and phytosterols in selected weeds extracts. Choudhary et al. (2014) also conducted the qualitative phytochemical screening of C. sativa by using extracts of four solvents viz; alcohol, chloroform, aqueous and n- hexane. In that study, he reported the presence and absence of different phytochemicals such as amino acids, proteins, flavonoids, tennis and steroids in different extracts of C. sativa. Lone and Lone (2012) also observed the protein in extract of C. sativa by subjecting the solvents to aqueous and acetone extracts. Girish (2017) states that the ethanolic, methanolic and aqueous extracts of L. camara showed various antimicrobial activities due to the presence of many bioactive pytocompounds in an In vitro study. Methanolic extracts of P. hysterophorus on the seed survival rate and seedling growth of Oryza sativa could be used to develop ecofriendly herbicide (Motmainna et al., 2021).
Lone and Lone (2012) reported that acetone extract in C. sativa, exhibited higher potential of antimicrobial activity by showing inhibition zone against fungi Cryptococcus neoforms, Candida albicans and bacteria Pseudomone aeruginosa, Vibo cholera. Malarkodi and Manoharan (2013) reported that ethanol, methanol and aqueous extracts of P. hysterophorus were tested In vitro for their activities against Candida albicans, Candida kefyr and Aspergillus niger with disc diffusion method. Among these extracts, methanol was one of the best solutions that showed higher antifungal activities. Aqueous extracts derived from L. camara poses the inhibitory effect in root and lateral root elongation rather than shoot elongation and germination in some agricultural crops (Ahmed et al., 2007).
CONCLUSION
In conclusion, the potential use of these selected weeds as a source of antagonists against A. solani offers an environmentally friendly strategy for early blight disease management. The secondary metabolites which are detected in this study, are also important because they frequently contribute to the therapeutic characteristics of plants and can aid in disease suppression by directly destroying fungal pathogens. Moreover, the findings of this research hold substantial significance in lowering reliance on synthetic pesticides. Farmers and other agricultural stakeholders may explore new biological strategies, from the development of sustainable and cost-effective disease management approaches as a result of this study.
REFERENCES
Adkins S., Shabbir A. , Dhileepan K. (2019). Parthenium weed: biology, ecology and management. CAB International.
Ahmed R., Uddin M.B., Khan M.A.S.A., Mukul S.A., Hossain M. K. (2017). Allelopathic effects of Lantana camara on germination and growth behavior of some agricultural crops in Bangladesh. Journal of Forestry Research, 18: 301-304.
Ali E.M., Almagboul A.Z., Khogali S.M., Gergeir U.M. (2012). Antimicrobial activity of Cannabis sativa L. Chinese Medicine, 3: 61-64.
Bais R.K., Ratan V., Kumar S., Tiwari A. (2019). Comparative analysis of various strategies for management of early blight of tomato incited by Alternaria solani (Ellis and Martin) Jones and Grout. The Pharma Innovation Journal, 8: 15-20.
Berardo M.E.V., Mendieta J.R., Villamonte M.D., Colman S.L., Nercessian D. (2024). Antifungal and antibacterial activities of Cannabis sativa L. resins. Journal of Ethnopharmacology, 318: 116839.
Choudhary N., Siddiqui M., Bi S., Khatoon S. (2014). Variation in preliminary phytochemicals screening of Cannabis sativa L. leaf, stem and root. Int. J. Pharmacogn., 1: 516-519.
Girish K. (2017). Antimicrobial activities of Lantana camara Linn. Asian Journal of Pharmaceutical and Clinical Research, 10: 57-67.
Gupta A., Naraniwal M., Kothari V. (2011). Modern extraction methods for preparation of bioactive plant extracts. International Journal of Applied and Natural Sciences, 1: 8-26.
Jones S., Baizan-Edge A., MacFarlane S., Torrance L. (2017). Viral diagnostics in plants using next generation sequencing: computational analysis in practice. Frontiers in Plant Science, 8: 1770.
Kato-Noguchi H., Kurniadie D. (2021). Allelopathy of Lantana camara as an invasive plant. Plants, 10: 1028.
Khan I.H., Javaid A. (2020). Antifungal activity of leaf extract of Cannabis sativa against Aspergillus flavipes. Pakistan Journal of Weed Science Research, 26: 447.
Lone T.A., Lone R.A. (2012). Extraction of cannabinoids from Cannabis sativa L. plant and its potential antimicrobial activity. Univers. J. Med. Dent., 1: 51-55.
Mallik I., Arabiat S., Pasche J.S., Bolton M.D., Patel J.S., Gudmestad N.C. (2014). Molecular characterization and detection of mutations associated with resistance to succinate dehydrogenase inhibiting fungicides in Alternaria solani. Phytopathology, 104: 40-49.
Malarkodi E., Manoharan A. (2013). Antifungal activity of Parthenium hysterophorus L. Journal of Chemical and Pharmaceutical Research, 5: 137-139.
Mandeel Q.A. (2020). Pathogenic and molecular characterization of isolated from infected tomato plants in Saudi Arabia. Saudi Journal of Biological Sciences, 21: 612-619.
Mao R. (2020). Dispersal and persistence of invasive parthenium weed: spread pattern, dynamics and a future perspective. PhD Thesis. The University of Queensland.
Mao R., T.L.T. Nguyen, O.O. Osunkoya, S.W. Adkins (2019). Spread pathways of the invasive weed Parthenium hysterophorus L.: The potential for water dispersal. Austral. Ecology, 44: 1111-1122.
Motmainna M., Juraimi A.S., Uddin M.K., Asib N.B., Islam A. M., Ahmad-Hamdani M.S., Hasan M. (2021). Phytochemical constituents and allelopathic potential of Parthenium hysterophorus L. in comparison to commercial herbicides to control weeds. Plants, 10: 1445.
Muriel Q., Moreels P. (2022). Etude des barrières de reproduction entre Solanum chilense et Solanum lycopersicum. Faculté des sciences, Université catholique de Louvain.
Nguyen T., Bajwa A.A., Belgeri A., Navie S., O’Donnell C., Adkins S. (2017). Impact of an invasive weed, Parthenium hysterophorus, on a pasture community in south east Queensland, Australia. Environmental Science and Pollution Research, 24: 27188-27200.
Olikh N., B.G. Nayyar, Serwer A., Ajmal M., Naseer A., Hashmi T.K. (2023). Phytochemical analysis and antifungal activity of selected medicinal plant extracts against Alternaria alternata. Pure and Applied Biology, 12: 1056-1065.
Pal G.K., Kumar B., Shahi S.K. (2013). Antifungal activity of some common weed extracts against phytopathogenic fungi Alternaria spp. International Journal of Universal Pharmacy and Life Sciences, 3: 6-14.
Pandey A., Tripathi S. (2019). Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. Journal of Pharmacognosy and Phytochemistry, 2.
Qu T., Du X., Peng Y., Guo, W., Zhao C., Losapio G. (2021). Invasive species allelopathy decreases plant growth and soil microbial activity. PLoS ONE, 16, e0246685.
Shaikh J.R., Patil M. (2020). Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud., 8: 603-608.
Thomma B.P. (2003). Alternaria spp.: from general saprophyte to specific parasite. Molecular Plant Pathology, 4: 225-236.